|
|
|
|
Individualized Quality Control Plan
 After January 1, 2016, the Centers for Medicare and Medicaid Services (CMS) will require clinical labs to revise their QC procedures on CLIA non-waived tests. After this date, labs will be required to choose between performing CMS/CLIA default guidelines or they must implement an Individualized Quality Control Plan (IQCP).
After January 1, 2016, the Centers for Medicare and Medicaid Services (CMS) will require clinical labs to revise their QC procedures on CLIA non-waived tests. After this date, labs will be required to choose between performing CMS/CLIA default guidelines or they must implement an Individualized Quality Control Plan (IQCP).
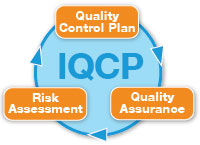
An IQCP consists of three key parts: (1) a Risk Assessment (RA) which identifies areas where errors or failures could occur in entire workflow path (pre-analytical, analytical, post-analytical); and assesses risk for harm to the patient if an error would occur and be reported; (2) a Quality Control Plan (QCP) which defines the control mechanisms in place for detecting or preventing errors; and finally (3) a Quality Assessment (QA), a process to continually monitor the efficacy of the QCP.
Resources such as frequently asked questions (FAQs) on how to set-up an IQCP can be found at CMS, College of American Pathologists (CAP) and additional resource materials are available through the American Society for Microbiology (ASM). In addition, a CAP IQCP eligibility determination flowchart as well as a CAP checklist for IQCP requirements are also available. Below are additional Hardy resources on IQCP FAQs from an interview with Dr. Susan E. Sharp and a customer notification letter on how these changes will affect Hardy's CLSI M22 exempt media.
For additional questions, please contact our Hardy Technical Services Department and 800-266-2222, option 2 or via email at TechService@HardyDiagnostics.com.
|
|

|

|

|

|
|
|
|
|
|
|
|