|
|
|
|

|

|

|
|
|
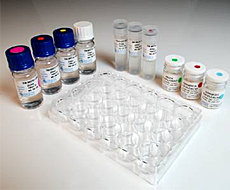 Hardy Diagnostics is pleased to present the new TB MODS TEST KIT™!
MODS = Microscopic Observation Drug Susceptibility
Hardy Diagnostics is pleased to present the new TB MODS TEST KIT™!
MODS = Microscopic Observation Drug Susceptibility
What is the TB MODS TEST KIT?
-
Detects the presence of viable
Mycobacterium tuberculosis
and
-
Performs susceptibility testing simultaneously and within the same procedure.
-
Detects resistance to
both
isoniazid and rifampicin; the two most commonly used drugs for therapy.
-
Uses liquid culture for accelerated growth.
-
Utilizes the TB cording phenomenon for identification, which is easily viewed with an inverted microscope.
-
Detects susceptible, mono-resistant, and multi-drug resistant (MDR) TB usually within a 5 to 10 day incubation period.
-
Can be used to monitor patients on therapy. Detects only living organisms.
-
Cost effective and labor saving at the price of approximately $5.50 per test.
-
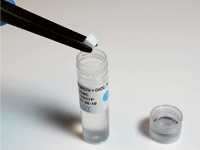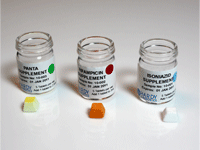
Ready-to-use antibiotics available in an easy-to-dissolve tablet. No measuring, weighing, or mixing needed.
-
All vials and reagents are color coded to simplify and error-proof the procedure.
-
Unique protective flexible silicone sealing lid for increased safety. Tray remains permanently sealed throughout the entire incubation and examination procedure. Safety lid can be easily pierced with a needle/syringe if sub-culturing or rapid speciation is necessary.
-
Formulated in a standardized, ready-to-use kit format complete with 24-well trays and color coded vials.
-
Kit contains all necessary components. No need to source from multiple vendors.
-
No expensive capital equipment required. Utilizes an inverted microscope (approximately $2,500 USD). See below.
-
The conventional MODS method was approved by the WHO for use in direct specimen testing of sputum for TB.
19
-
The WHO determined the conventional MODS method to be 98% sensitive and 99% specific for the detection of rifampin resistance; and 91% sensitive for isoniazid resistance.
19
-
TB MODS Kit is a BSL-2 Kit (Biological Safety Level - 2), not BSL-3.
-
TB MODS is often used as a follow-up test to PCR. Positive PCR tests for rifampin resistance must be followed up with
a culture susceptibility test for isoniazid.
-
Not currently for sale within the United States.
|

|

|
|

|

|

|
|

|
|
|

|
|
|
|

|

|
|
|
TB Mods Test Kit Antibiotics
|
Ready-to-use format
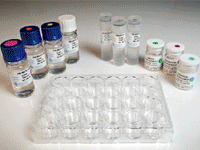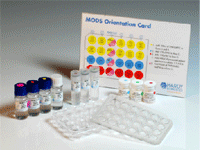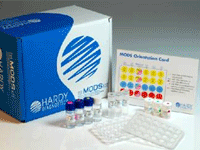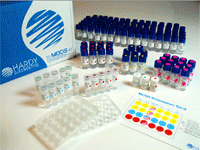
|
Protective Sealing Lid

|

|

|
|

|

|

|
|

|
|
|
|

|

|
|
|
-
Abstract of a study to compare the performance of Hardy MODS Kit (kMODS) to conventional MODS (cMODS) culture in a clinical setting.
A diagnostic evaluation of the Hardy MODS kit for the diagnosis of TB and MDR-TB from sputum samples.
-
An evaluation of the MODS assay for the diagnosis of TB in children in Vietnam showing that infection detection rates were significantly higher for MODS testing than for the MGIT method.
Microscopic Observation Drug Susceptibility assay (MODS) for early diagnosis of tuberculosis in children.
-
A study comparing the sensitivity, specificity, and time to results for four direct susceptibility methods compared to conventional indirect testing for detection of resistance to rifampicin and isoniazid in
M.tuberculosis
.
Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis.
-
A study showing the MODS method as a rapid, low-cost, low-tech tool for high-performance detection of TB and MDRTB as compared to competing methods hampered by low sensitivity (sputum smear), long delays (solid media culture), high cost (automated liquid culture methods), and lengthy subculture to determine susceptibility (MDRTB).
The MODS method for diagnosis of tuberculosis and multidrug resistant tuberculosis.
-
A study documenting the speed and accuracy of the MODS assay in diagnosing Tuberculous meningitis (TBM), compared to the low sensitivity of smear microscopy (CSF) and PCR and the infrastructure and expense required for molecular testing.
Evaluation of the MODS culture technique for the diagnosis of tuberculous meningitis.
-
An evaluation of MODS as an alternative method for directly identifying MDRTB in sputum samples in a timely and affordable way in resource-limited settings.
Evaluation of Microscopic Observation Drug Susceptibility assay for detection of multidrug-resistant
Mycobacterium tuberculosis
.
-
A study showing that MODS culture from sputum offers more rapid and sensitive detection of TB and MDRTB than existing gold-standard methods.
Microscopic Observation Drug Susceptibility assay for the diagnosis of TB.
-
Documentation of MODS as a novel assay to detect TB and MDRTB directly from sputum samples inexpensively, rapidly, and effectively in resource-poor settings.
Microscopic Observation Drug Susceptibility assay, a rapid, reliable diagnostic test for multidrug-resistant tuberculosis suitable for use in resource-poor settings.
-
A study showing that the MODS assay was as accurate as and more rapid than the reference 7H10 agar method.
Performance of the Microscopic Observation Drug Susceptibility assay in drug susceptibility testing for
Mycobacterium tuberculosis
.
-
Study highlighting the suitability of MODS as a rapid, inexpensive, sensitive, and specific method for TB detection and susceptibility testing in countries burdened by high infection rates and increasing multiple-drug-resistant cases compared to PCR and MABA techniques.
Rapid, efficient detection and drug susceptibility testing of
Mycobacterium tuberculosis
in sputum by microscopic observation of broth cultures. The Tuberculosis Working Group in Peru.
-
An evaluation of the MODS assay for the direct detection of
Mycobacterium tuberculosis
isoniazid and rifampin drug resistance.
Clinical evaluation of the Microscopic Observation Drug Susceptibility assay for detection of
Mycobacterium tuberculosis
resistance to isoniazid or rifampin.
-
A study showing that the MODS method is useful in reducing the global inequity in TB diagnostics for TB control and patient care in resource-limited settings.
Introducing MODS: a low-cost, low-tech tool for high-performance detection of tuberculosis and multidrug resistant tuberculosis.
-
A study demonstrating that MODS produced a greater yield and faster recovery of
M. tuberculosis
from children with suspected pulmonary tuberculosis than traditional methods, significantly improving local capabilities to detect pediatric tuberculosis in resource-poor settings.
Improved recovery of
Mycobacterium tuberculosis
from children using the microscopic observation drug susceptibility method.
-
A collection of duplicate gastric-aspirate specimens from high-risk children showed that MODS culture was the best available diagnostic test for pulmonary tuberculosis compared to traditional culture and PCR methods.
Diagnostic approaches for paediatric tuberculosis by use of different specimen types, culture methods, and PCR: a prospective case-control study.
-
A call to provide a more consistent approach to TB diagnostic methods in children in response to the global emergence of drug-resistant tuberculosis in high tuberculosis-endemic settings.
Research into tuberculosis diagnosis in children.
-
A systematic review and meta-analysis into the accuracy of MODS and thin layer agar (TLA) assays as effective and inexpensive alternative methods of TB detection for rapid screening of patients at risk of drug-resistant tuberculosis.
Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis.
-
An evaluation of MODS as a cost-effective, sensitive, specific and rapid test appropriate for the detection of HIV-associated TB in resource limited settings.
Diagnosis of pulmonary tuberculosis by microscopic observation drug susceptibility assay in HIV positive patients.
-
WHO report (2010) of
The Global Plan to Stop TB 2011-2015
initiative showing the MODS method as a target for sustainable adoption.
The Global Plan to Stop TB 2011-2015
: transforming the fight towards elimination of tuberculosis.
-
WHO report (2009) on the
Stop TB Partnership, Pathways to Better Diagnostics for Tuberculosi
s initiative showing the MODS method under evaluation as a viable new method for TB detection.
Pathways to better diagnostics for tuberculosis: a blueprint for the development of TB diagnostics.
-
WHO report (2010) on
Non-Commercial Culture and Drug Susceptibility Testing Methods for Screening of Patients at Risk of Multi-Drug Resistant Tuberculosis
documenting the revised and standardized MODS platform as an alternative method for reference laboratory testing compared to conventional methods.
Non-commercial culture and drug-susceptibility testing methods for screening of patients at risk of multi-drug resistant tuberculosis.
-
Findings on the MODS assay as a rapid, simple, economical, and feasible method for simultaneous culture and drug susceptibility testing of TB in extra-pulmonary TB cases.
Utility of Microscopic Observation of Drug Susceptibility (MODS) assay for
Mycobacterium tuberculosis
in resource constrained settings.
-
A promising performance of the MODS assay in a cohort of predominantly HIV-infected TB suspects from South Africa in support of expanding MODS to similar settings in sub-Saharan Africa.
Rapid diagnosis of tuberculosis and multidrug resistance by the microscopic observation drug susceptibility assay.
-
An excerpt from the 2012 Unitaid "Tuberculosis Diagnostic Technology Landscape".
The MODS assay was developed as a faster, cheaper, and more sensitive test than other solid or liquid culture-based tests currently in use for TB diagnosis.
-
A research article from www.plosone.org "MODS for MDR-TB Suspects in Zimbabwe".
Microscopic-Observation Drug-Susceptibility Assay for
the Diagnosis of Drug-Resistant Tuberculosis in Harare, Zimbabwe
-
Research group recommends MODS.
The TB MODS Kit was deemed to be both rapid and accurate for identification and sucseptibility testing.
-
In an effort to update and clarify policies on tuberculosis drug susceptibility testing (DST), the World Health Organization (WHO) commissioned a systematic review evaluating WHO-endorsed diagnostic tests.
Diagnostic accuracy and reproducibility of WHO-endorsed phenotypic drug susceptibility testing methods for first-line and second-line anti-tuberculosis drugs: a systematic review and meta-analysis.
-
A field evaluation of the Hardy TB MODS Kit™ for the rapid phenotypic diagnosis of tuberculosis and multi-drug resistant tuberculosis.
The results of this evaluation and the improvement on procurement and biosafety issues provide an important opportunity to use wide scale introduction of the MODS kit as a vehicle for greatly expanding equitable access to first and second line DST in settings where the need is greatest.
|

|

|
|

|

|

|
|

|

|

|

|
|
|
 At Hardy, you will find a complete selection of laboratory supplies including clinical and industrial culture media, rapid test kits, stains, reagents, dehydrated culture media, animal blood products, antisera, proficiency testing programs, anaerobic gas generators, specimen swabs and transports, dilution vials, QC microorganisms, susceptibility disks, immunology kits, pathogen detection kits (Salmonella, E. coli O157, Listeria, MRSA, Staph), Petri plates, agar based prepared media and broths, chromogenic media, mastitis media, incubators, environmental monitoring supplies, parasitology and mycology supplies, custom media formulations, disposable gloves, labcoats, loops and much more!
At Hardy, you will find a complete selection of laboratory supplies including clinical and industrial culture media, rapid test kits, stains, reagents, dehydrated culture media, animal blood products, antisera, proficiency testing programs, anaerobic gas generators, specimen swabs and transports, dilution vials, QC microorganisms, susceptibility disks, immunology kits, pathogen detection kits (Salmonella, E. coli O157, Listeria, MRSA, Staph), Petri plates, agar based prepared media and broths, chromogenic media, mastitis media, incubators, environmental monitoring supplies, parasitology and mycology supplies, custom media formulations, disposable gloves, labcoats, loops and much more!
|
|
|